Improving Understanding of Hydrogen Bonding for Thermodynamic Modeling

Aseel Bala is a chemical engineering Ph.D. candidate at Michigan State University, conducting research with Dr. Carl Lira on the thermodynamic phase behavior of systems with hydrogen bonding. The work aims to improve understanding of hydrogen bonding in collaboration with industry partners from Dow Chemical, FLUOR Corporation, and Honeywell UOP, and focuses on mixtures including water, alcohols and organic acids. While this project has far-reaching impacts in multiple fields including biology, plastics and even drug design, the effects are especially timely in the chemical and fuel industries.
Fossil fuels like petroleum are the primary sources of fuels and chemicals today. We use them to power our cars and to heat and cool our homes. The manufacturing of products like chemicals and plastics, from non-renewable energy sources such as crude oil, has been optimized over decades. Engineers can model the phase behavior of petroleum components (which do not hydrogen bond) well and can therefore efficiently produce affordable products at industrial scales. In contrast, the separations of chemicals derived from bio-renewable energy crops like corn, grain and sugar cane, are complicated because water and intermediate products form hydrogen bonds. Existing thermodynamic models struggle to accurately capture the phase behavior of such systems. Therefore, significant experimentation must be conducted to collect thermodynamic data before designing, constructing and operating units such as reactors and distillation columns. Ultimately, this leads to extended pilot plant testing and makes development more expensive and slower compared to petroleum-based processes. Aseel and her collaborators are focused on improving the traditional thermodynamic models used in process design.
Aseel’s work combines three complementary tools. The first tool is a flexible thermodynamic model she and her advisor developed that realistically incorporates hydrogen bonding. The second tool is spectroscopic analyses, specifically IR and NMR spectroscopy, which are used to determine the parameters for the thermodynamic model. The third tool is quantum and molecular mechanics (QM/MM) simulations that are leveraged to guide interpretation of the complex experimental spectra. Aseel and her colleagues have successfully integrated their model as a user model into AspenPlus® – the leading process simulation software package used by engineers in industry.
One of the technical obstacles the group faced involved interpreting molecular behavior from experimental spectra. In IR spectroscopy, molecules are excited with infrared light and the amount of light absorbed by the sample is detected. Absorption of light at different wavelengths induces characteristic vibrations of different bonds within the molecule and analysis of the resulting spectra yields information about the concentrations of certain functional groups in the solution. The region of the spectra that is of most significance for this work is the broad hydroxyl band. The band breadth occurs because of the variety of hydrogen bonding in solution. Interpretation of the broad IR band is nontrivial; the band has historically been used for qualitative information. Studies quantifying the hydroxyl band have been limited and somewhat speculative. However, Aseel and her colleagues were able to develop a new method which uses quantum chemical calculations to quantify hydrogen bonding species concentrations from IR spectra for alcohols.
Aseel explains that progress in this project could not have been accomplished without the extensive range of software licenses and large-scale computing resources on MSU’s HPCC. Moreover, the consultants at ICER provided critical expertise that extended from the computer science at the surface to the underlying chemical and physical phenomena. Specifically, Dr. Chun-Min Chang was always eager to help Aseel navigate the computational tools and design her calculations to yield meaningful results in an efficient manner.
As she nears graduation, Aseel is especially appreciative of the people who supported her research here at MSU, particularly Dr. Carl Lira, who was and continues to be a consistent source of support and inspiration, and Ph.D. student Bill Killian. Bill has worked extensively with Aseel on interpreting spectroscopy and will continue exploring the project’s future directions. Undergraduate research assistants Jackson Storer and Renming Liu have also made valuable contributions to the work. MSU’s campus has also provided Aseel with access to a diverse range of experts, such as Dr. James E. (Ned) Jackson and Tayeb Kakeshpour in the department of chemistry, who make collaboration exciting and fruitful.
Aseel’s ultimate goal, like others in the field of thermodynamics, is “to remove obstacles that make the design of biofuels and biochemical processes less efficient compared to petroleum-derived chemicals.” She is excited that her research will not just stay on paper but is put to practical use by those in the chemical industry. She hopes her work will contribute to improved processes in the biorenewable energy sphere, reducing the design costs enough to make biobased products price-competitive with those from petroleum.
Figure 1 Figure 2
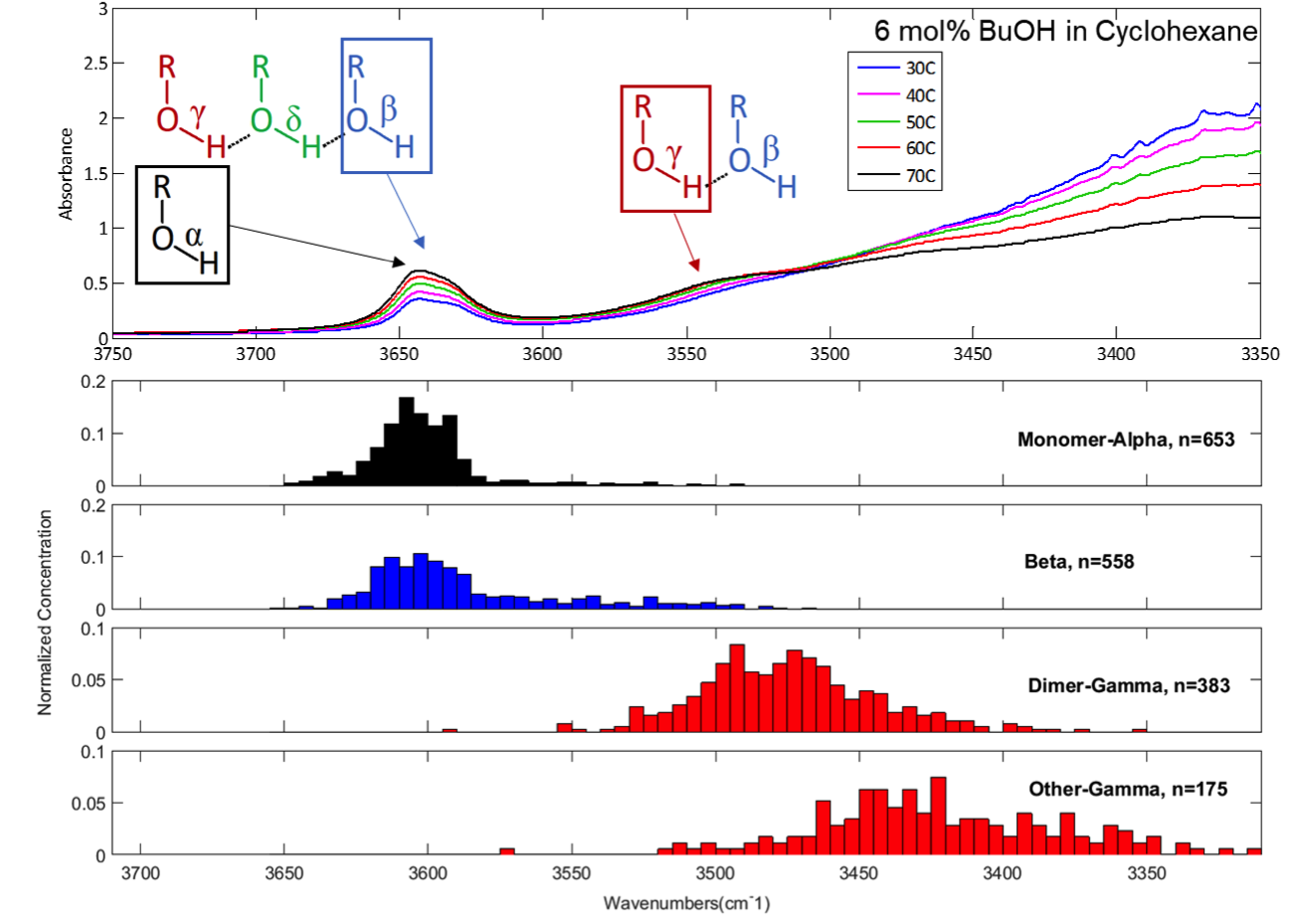
Figure 1- Summary of research objectives: This work combines three approaches to improve thermodynamic representation of hydrogen bonding systems: the theoretical model, IR and NMR spectroscopy and quantum mechanics simulations.
Figure 2 – Top: Infrared spectrum of a 6 mol% butanol in cyclohexane mixture. Bottom: Quantum mechanical calculations of the infrared vibrational frequencies of different types of OH bonds. Using frequencies calculated from simulations, we aim to deconvolute peaks in experimental infrared spectra.
