Applying Genomics to Develop an Understanding of Uterine Cancer

Jake Reske is a graduate student at Michigan State University’s biomedical research center in Grand Rapids. He studies Genetics and Genome Sciences in the department of Obstetrics, Gynecology & Reproductive Biology (OBGYN) and works under Dr. Ronald Chandler. More specifically, a major focus of his research is endometrial cancer, or cancer that begins in the uterus. Reske’s interest and passion for cancer research bloomed during his undergraduate and pre-graduate years. As a genetics major on the pre-med track, he became intrigued by the idea of pursuing a career path in biomedical research. This led him to join a biochemistry lab during his undergraduate degree, followed by a cancer lab engaged in genetic work with mice as a post-baccalaureate researcher. The latter experience catalyzed his love for genetics and solidified his desire continue to do cancer research.
 Reske explains that his work seeks to discern “why we see very specific patterns of mutation in uterine cancer that are always coming up in patients.” Essentially, researchers have observed the very same mutations in roughly 20-25% of endometrial cancer patients. This work seeks to understand why that occurs at a molecular level and how these mutations relate to the development of endometrial cancer.
Reske explains that his work seeks to discern “why we see very specific patterns of mutation in uterine cancer that are always coming up in patients.” Essentially, researchers have observed the very same mutations in roughly 20-25% of endometrial cancer patients. This work seeks to understand why that occurs at a molecular level and how these mutations relate to the development of endometrial cancer.
The research Reske does is unique and particularly compelling because it integrates traditional wet-laboratory work and new, cutting-edge computational techniques. These two approaches work together—first by inducing mutations in mice and then using computational genetic techniques to understand the biology involved in the observations and results of tests on mice. These approaches are permitted by new high-throughput genetic sequencing technologies invented in the past 10-15 years. Genetic sequencing involves taking a cell or tissue and mapping the DNA within the sample. This DNA can then be analyzed using high-performance computing systems for patterns and relationships between changes in the tissue and mutations in the genes.
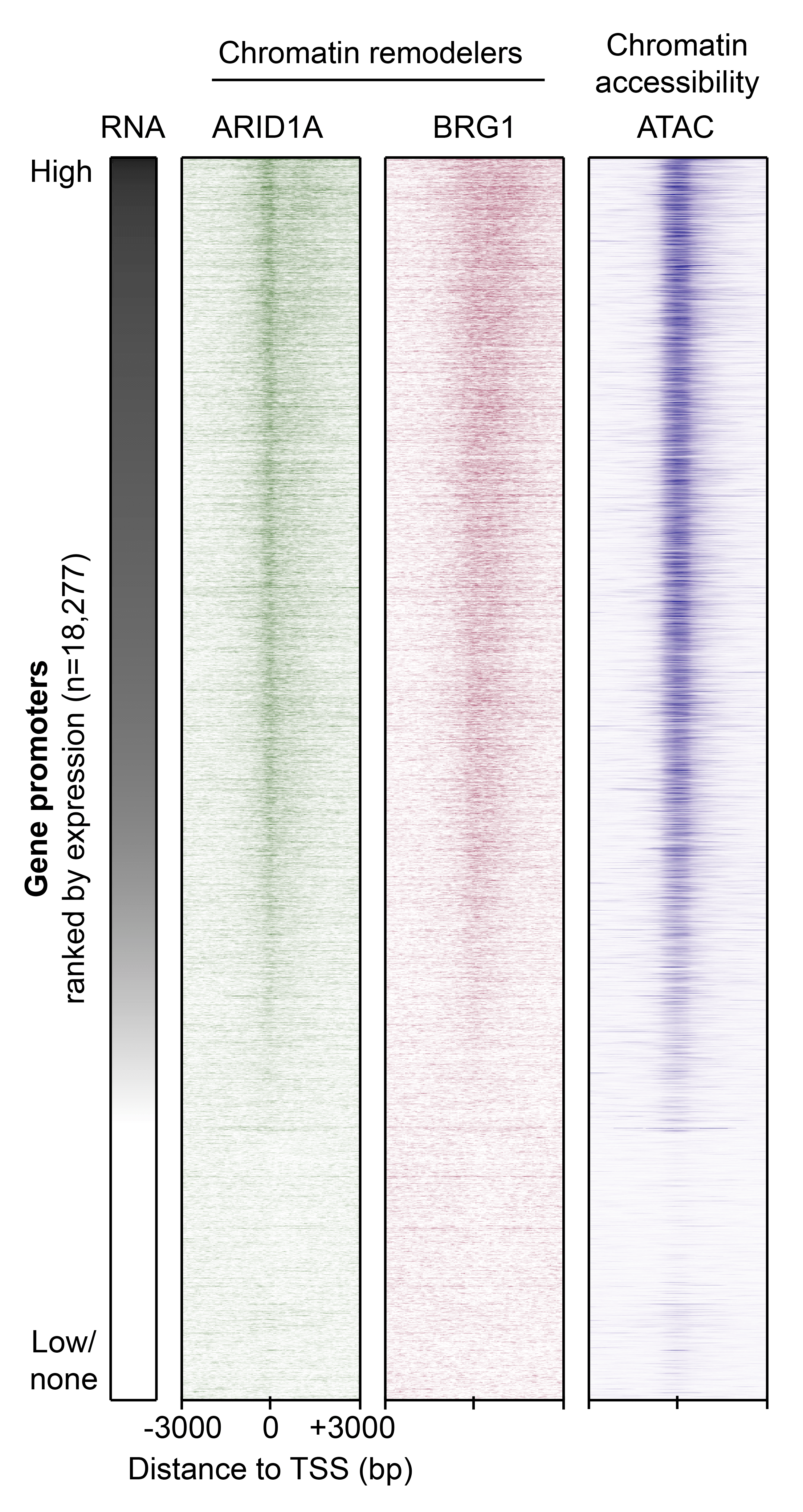
An example of how this work manifests is highlighted in the figure shown here. These approaches allow Reske to measure molecular features across all genes in the genome with high-throughput techniques. Pictured is a snapshot of gene expression, cancer-relevant protein interactions, and DNA accessibility at more than 18,000 genes in human endometrial epithelial cells, where darker colors indicate more signal is observed for that gene. From this analysis, Reske is able to conclude that the proteins ARID1A and BRG1 are associated with highly expressed and accessible genes in the endometrium.
While the integration of traditional laboratory work and advanced computational studies provides many new opportunities for researchers, the process does have its challenges. It has proven incredibly difficult for Reske's team to use these new techniques to determine how DNA is packaged within cells. This is because one of the genes Reske studies (ARID1A) is directly involved in packaging DNA (i.e. determining the way genes are organized and folded to fit into a cell). Thus, mutations in genes that affect the organization and structure of DNA, like ARID1A, make it difficult to analyze DNA itself—as they alter the DNA, rendering it abnormal and therefore difficult to interpret. This means Reske must utilize a brand new computational and laboratory technique to measure how this particular DNA is packaged, known as the Assay for Transposase-Accessible Chromatin, or ATAC-sequencing.
The computational techniques Reske uses in his research would be unviable if not for HPCC services. These techniques involve sequencing and analyzing the 3 billion base pairs[1] contained within the human DNA. If we were to attempt to print out and analyze each individual base pair, it would be very costly and take an extraordinary length of time. For example, the Human Genome Project achieved this traditionally and it not only cost $3 billion, but took the researchers 13 years (1990-2003) to accomplish what is now much more achievable with HPCC. Reske states that now we can “achieve similar accuracy for about $1,000 in 24 hours.” These HPCC services allow Reske to utilize Genomics techniques efficiently and within a reasonable timeframe.
Ideally, this research will push cancer treatment in the direction of personalized medicine. Reske explains that “the whole premise of personalized health care and precision medicine is that instead of using the same drug to treat all cases of uterine cancer, we’ll look at patients’ genetics and identify which patients have which mutation and treat them with different medication accordingly.” Reske’s research will push the biomedical field toward this goal by providing the community with an understanding of what happens in a patient’s cells when they have the specific mutations that Reske studies. It will also demonstrate how their tissues differ from uterine cancer patients who do not have the same mutations. Simultaneously, gaining a deep understanding of how this type of uterine cancer operates creates opportunities for researchers to develop treatments that will be especially effective for patients with these specific mutations. These advancements in understanding human biology and in the biomedical field hold enormous potential to save many lives.
For a recent paper on this research led by Reske, Chandler, and post-doctoral researcher Mike Wilson, see "ARID1A and PI3-kinase pathway mutations in the endometrium drive epithelial transdifferentiation and collective invasion" published in Nature Communications.
[1] Base pairs are the building blocks of DNA.
